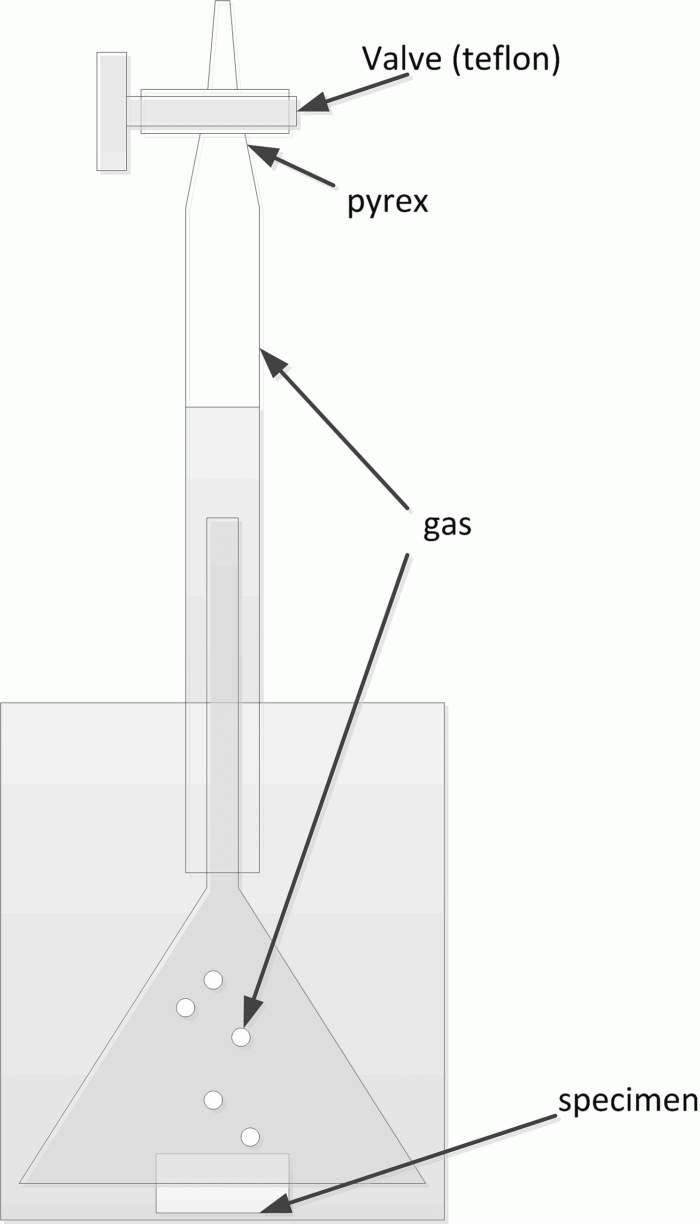
Two postgraduates (each in the framework of his/her research) had to compare corrosion rates of magnesium alloys in salt water measured by several techniques. Both obtained weird results, which can be summarized as follows:
- Four techniques provided corrosion rates on the order of magnitude of 1-5 mm/year. The fifth technique, namely the measurement of gas evolution in the course of corrosion, provided the corrosion rate of about 30 mm/year, which is unusually high.
- At the daytime the average corrosion rate measured by the gas evolution was significantly (more or less four times) lower than that measured at night.
These results were readily reproducible so that we had to find an explanation to this strange phenomenon.
Having excluded the possibility of ghosts, security officers and other supernatural factors, we considered the influence of temperature, atmospheric pressure and humidity. All these are different at night and during the day. Because measuring the temperature was the easiest way, we started from this and found that the difference between the day and the night could be as high as 10oC.
Normally, corrosion is faster at a higher temperature. Therefore, our phenomenon could not be explained from the point of view of the energy of activation. There was some chance that the mechanism of corrosion, too, could be influenced by the temperature change, but we decided not to consider this at the first stage.
The change of viscosity had to be ruled out, too, because at night the solution was more viscous and the gas evolution should be then lower at night.
We did not know what the influence of humidity could be and assumed that there was no influence.
The pressure could have some influence. Fortunately, some guys from another research group registered the atmospheric pressure two times a day. We looked at their data and found out that there was almost no difference between a day and a night.
In an ideal system the manometric pressure of the gas collected in a burette should be exactly equal to the atmospheric pressure. In reality, there was some inertia and a measurement performed early in the morning could still be influenced by the night temperature accumulated by the electrolyte. This influence could be evaluated for an ideal gas (pV = nRT) and if this was the case, we could expect “a morning distortion” in the gas volume of about 5%. This was much less than what we observed. Moreover, such a theory might be able to explain the first measurement early in the morning, but not the other measurements made at later hours.
Well, we understood that we got stuck. It is always very useful to get stuck, because then we started to check all parts of the system and soon found that our burettes were leaking. All of them. We bought new burettes, checked them for leak and the corrosion rates measured by the gas evolution started to be consistent with those measured by the other techniques.
Yet, we wanted to understand why night gas evolution measured at leaky burettes was always higher than in the daytime. The answer was simple: the thermal expansion coefficient of Pyrex (burette body) is 4×10 -6 K -1, while that of Teflon is 135×10-6 K-1. According to our evaluation, it means that if there was some 0.1 mm clearance between Pyrex and Teflon at the day, at night this clearance grew to 0.13 mm due to the fact that Teflon contracted much more than Pyrex. This is why it was leaking much more.
